The Swansea Baby Study
Probiotics in the prevention of eczema: a randomised controlled trial
SUMMARY
454 mother/baby pairs received either 10 billion Lab4B probiotics (220) or matching placebo (234) daily in the last trimester of pregnancy and for the first six months of infancy.
There was a significant reduction in the development of atopic eczema in the infants supplemented with Lab4B probiotics from birth.
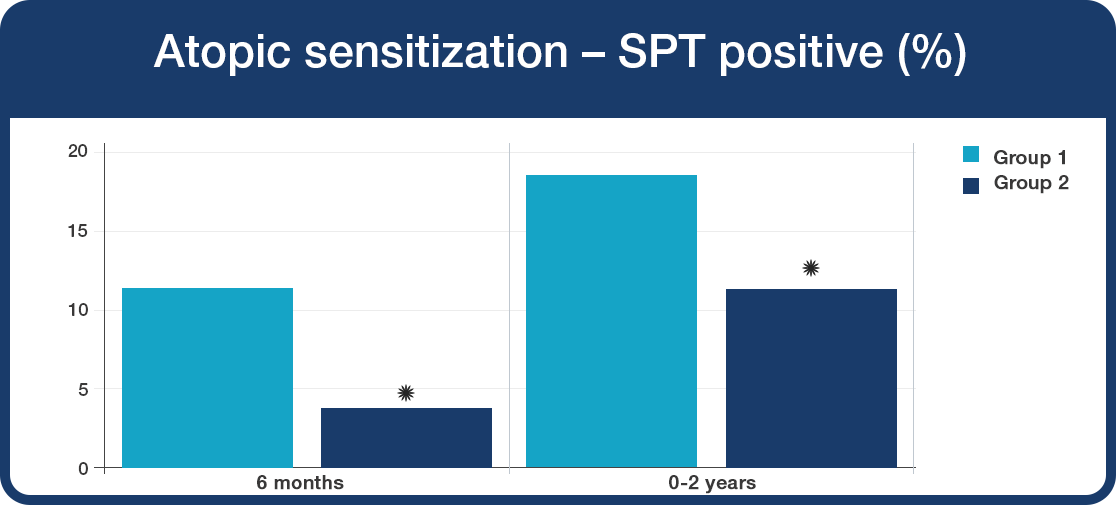
Development of atopic sensitisation to common allergens was significantly reduced with Lab4B supplementation.
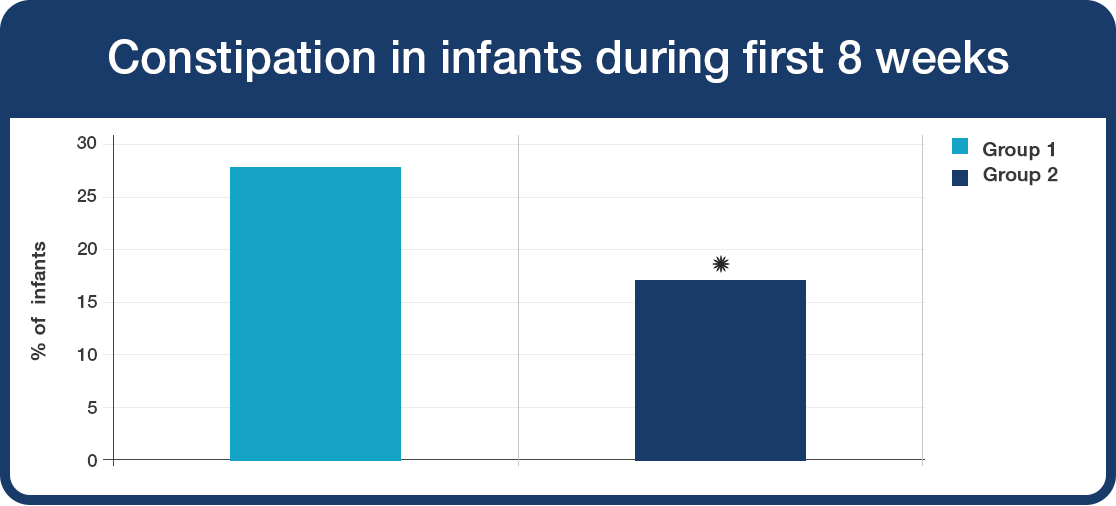
Constipation occurred in fewer infants supplemented with Lab4B probiotics.
Aim
This large randomised, double-blind, placebo-controlled study was designed to evaluate whether Lab4B probiotics given during infancy could prevent allergy in children.
Method
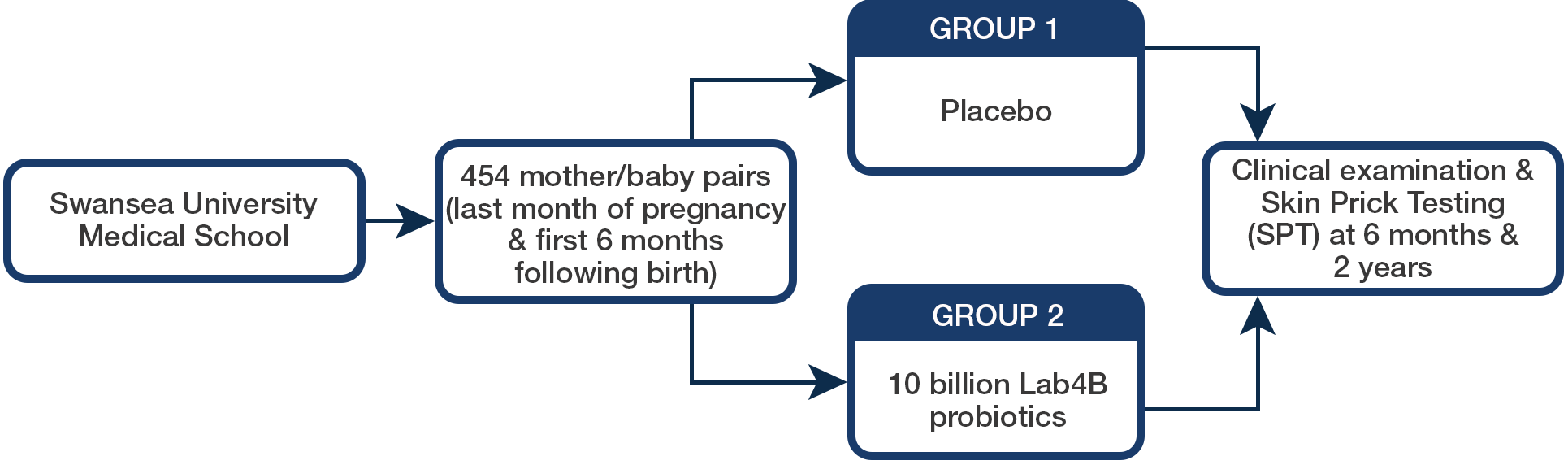
Results
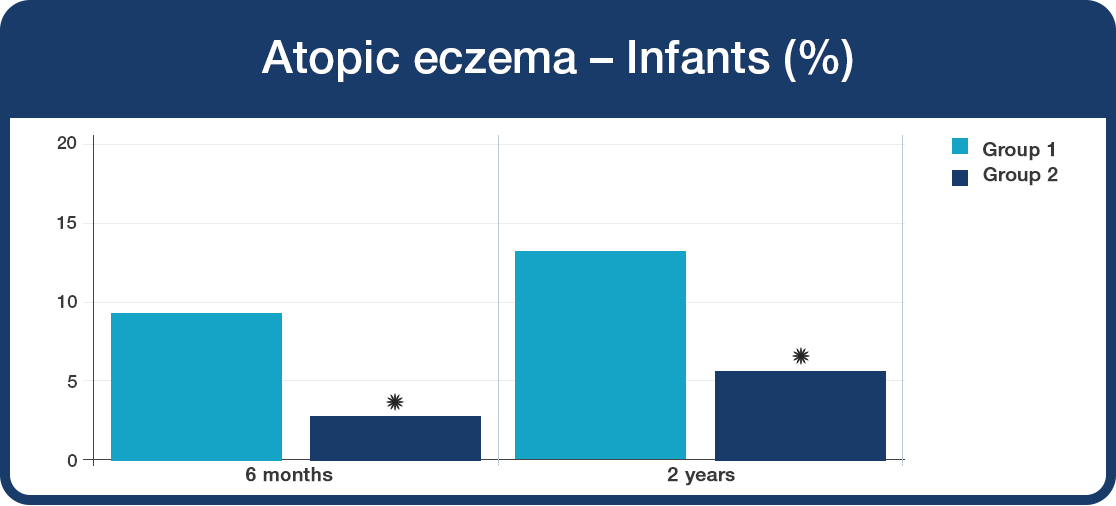
- The babies given the Lab4B probiotics (Group 2) were 57% less likely to develop atopic eczema than those in Group 1 (*P<0.05).
- The babies given Lab4B (Group 2) were 44% less likely to develop allergic reaction to common allergens, including pollen, cow’s milk, egg and house dust mite (*P<0.05).
- Significant reduction in constipation in babies during first 8 weeks of Lab4B probiotics use (Group 2) compared to Group 1 (*P<0.05).
Conclusion
The primary author, Prof. Steve Allen, concluded the following key message from the trial: ‘Lactobacilli and Bifidobacteria administered to pregnant women and infants aged 0-6 months prevented atopic sensitization and atopic eczema.’

