The Cambridge Clostridium difficile Study
Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea
SUMMARY
This double-blind, placebo-controlled study examines the role of probiotic administration in the prevention of Clostridium difficile-associated diarrhoea (CDAD) in elderly patients receiving antibiotic therapy.
Results showed that Lab4 probiotics reduced the incidence of Clostridium difficile diarrhoea in these patients.
46% of patients supplemented with Lab4 probiotics were positive for Clostridium difficile toxin compared to 78% of patients in the placebo group
Aim
This randomised, double-blind, placebo-controlled study was designed to provide evidence that supplementing with Lab4 probiotics while taking antibiotics can prevent the incidence of antibiotic-associated C. difficile diarrhoea (CDAD).
Method
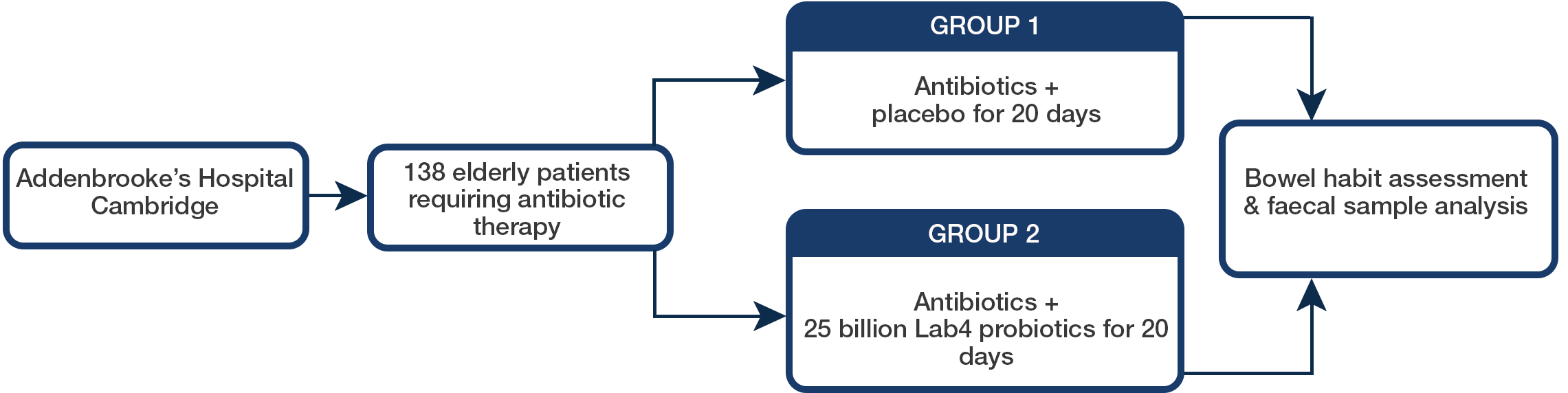
The trial took place at Addenbrooke’s Hospital in Cambridge, UK and involved 138 elderly patients requiring antibiotic therapy – 69 in the probiotics group and 69 in the placebo group. Measurements included both bowel habit assessment and faecal sample analysis.
Results
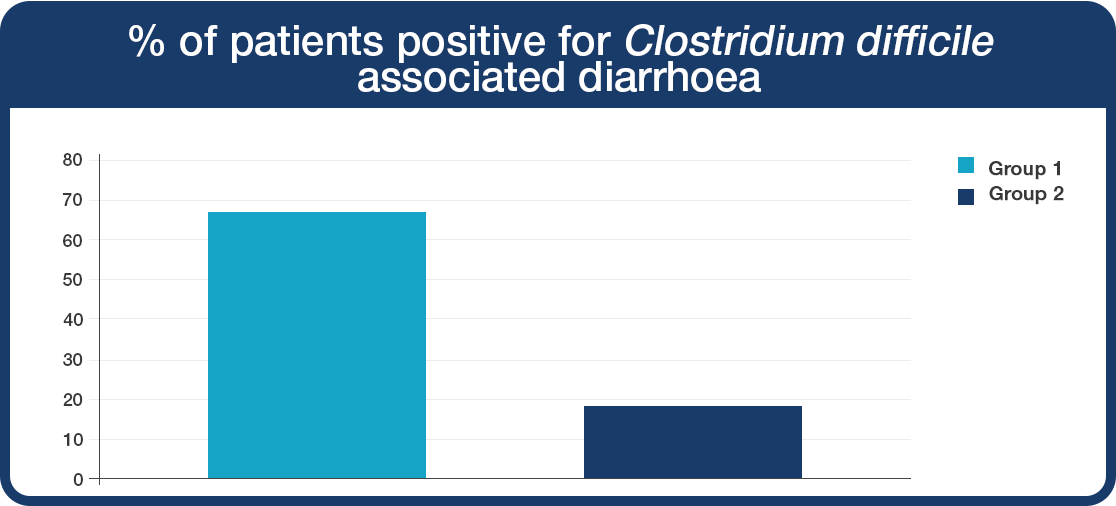
The patient group given Lab4 probiotics (Group 2) experienced a lower incidence of Clostridium difficile diarrhoea compared to the placebo (Group 1).
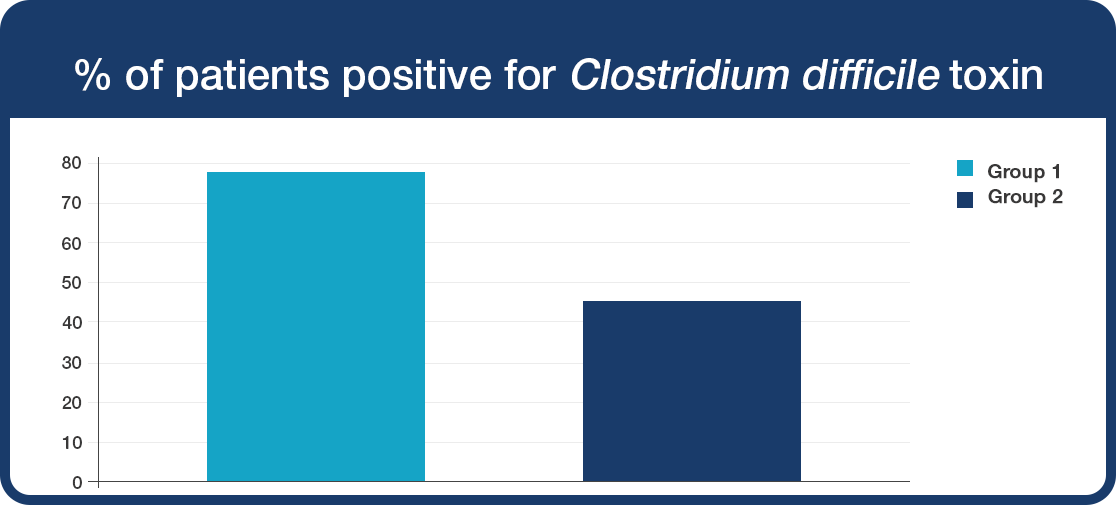
This effect was due to a reduction in the presence of the Clostridium difficile toxin in Group 2.
Conclusion
Supplementation with Lab4 probiotics can reduce the incidence of C. difficile diarrhoea in hospitalised patients.

